Projects
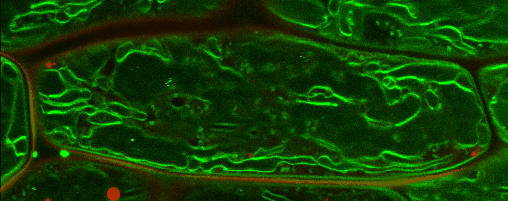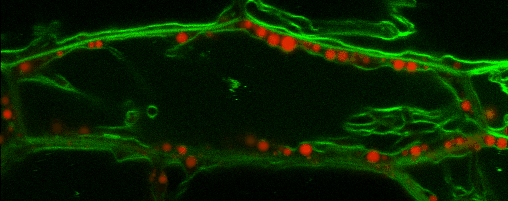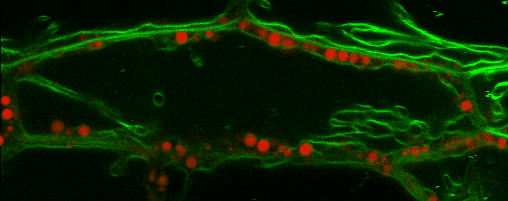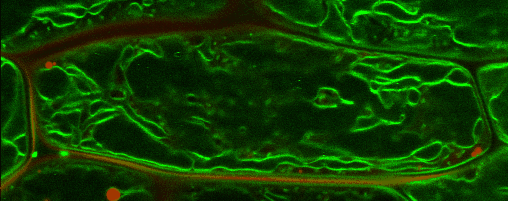
Lipid droplets
Lipid droplets (LDs) are special organelles consisting of a phospholipid monolayer surrounding a core of hydrophobic compounds, predominantly triacylglycerol (TAG). They are very prominent in seeds of angiosperms or the tapetum and the cytosol of pollen grains, but principally occur in all plant tissues in varying numbers.
For many years, LDs were regarded to be mere carbon storage organelles that provide energy (e.g. for heterotrophic growth stages like seed germination and seedling establishment). In the last years, however, it became more and more evident that these underrated organelles take an active part in many cellular processes. Yet, numerous questions about LD biology and their cellular roles remain to be resolved. One reason for this poor understanding of LDs is the small number of proteins actually known to reside there. While hundreds of potential candidates have been found in proteomic studies of lipid droplets from higher plants and algae, these were to the largest part never validated using a second line of evidence.
In our group, we therefore combine quantitative proteomics of LDs and specialized cell biological techniques developed in our lab to identify and verify potential candidates. In addition, the functional characterization of these identified proteins poses a big part of our research. For this, we make use of different genetic tools (e.g. CRISPR/Cas9) as well as biochemical (LC/MS-based proteomics), bioanalytical (different mass spectrometric analyses) and cell biological approaches (e.g. confocal laser scanning microscopy).
Pollen
Pollen development and pollen tube growth are delicate processes that are easily disturbed by stress. Pollination and proper pollen tube growth are crucial for plant reproduction, as they can limit fruit formation. We are particularly interested in the signaling underlying polar tip growth of pollen tubes.
Additionally to their functions in plants, pollen is an important feed for pollinators, such as bees. In this context, there have been millions of years of coevolution and it can be speculated that the pollen metabolome evolved to sustain a healthy pollinator population. Regarding this, we are interested in changes in the proteome, metabolome and lipidome throughout pollen development and pollen tube growth.
Moreover, pollen grains can easily be transiently transformed by particle bombardment and the growing pollen tubes can then be cultivated in vitro for cell biological studies (e.g. confocal laser scanning microscopy).

Plant-Pathogen Interactions
As part of the IRTG 2172 PRoTECT we are working on plant-pathogen interaction. For this, we study cell wall modifying enzymes and lipid droplet-associated lipases that might be involved in pathogen responses.

Analysis of small metabolites
The analysis of small metabolites by GC-MS can cover a broad variety of metabolites including sugars, phosphosugars, organic acids, amino acids, sterols and secondary metabolites. In addition, stable isotope labeling can be used to investigate metabolic fluxes.
In collaborative and own projects, we use GC-MS to determine the products of enzymatic reactions or to analyze metabolites in e.g. plant leaves, roots, tubers and pollen, algae, bacteria, mouse blood and brains, and mammalian cell culture. Our work on the GC-MS is part of the Göttingen metabolomics and lipidomic platform.


